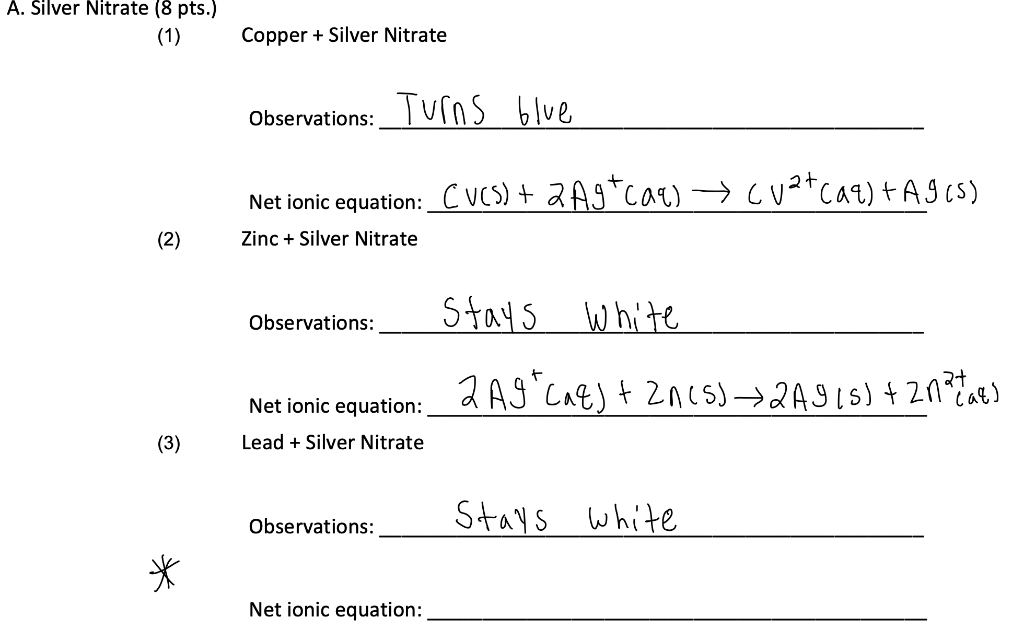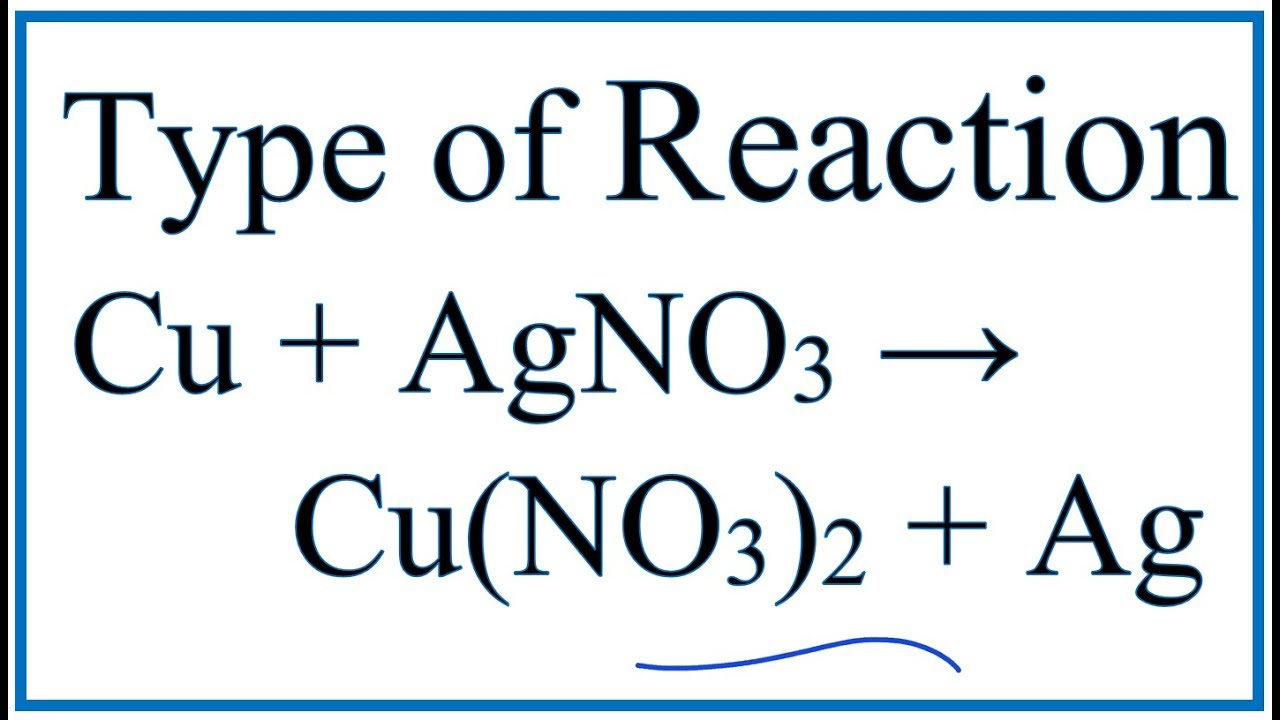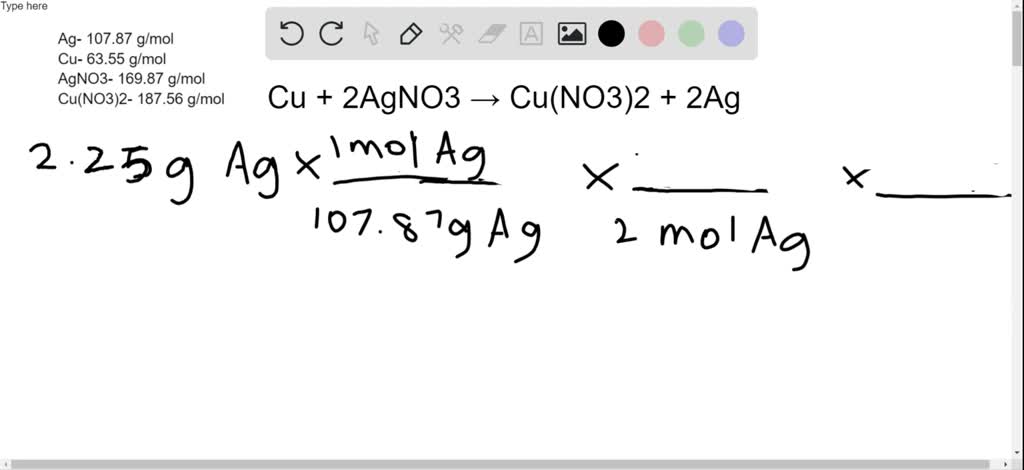
SOLVED:Copper reacts with silver nitrate through single replacement. a. If 2.25 g of silver are produced from the reaction, how many moles of copper(II) nitrate are also produced? b. How many moles

In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. Write down the reaction involved.

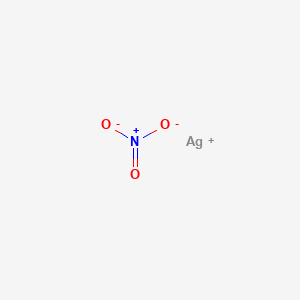
When copper is dipped in the solution of silver nitrate, the solution turns blue. Give the reason along with chemical equation?


/copper-wire-immersed-in-silver-nitrate-causing-blue-colour-81991997-582f14595f9b58d5b1a9b484.jpg)